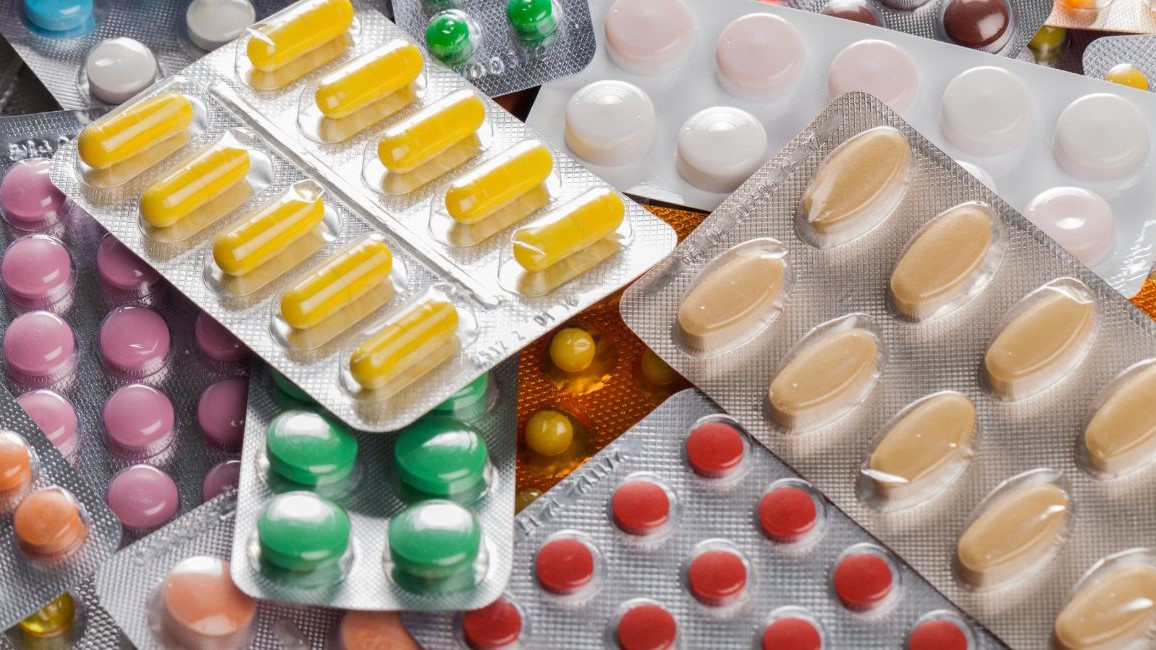
Ensuring the integrity and safety of products is a crucial concern for pharmaceutical companies and consumers in today’s medicine market. To address the growing risks of counterfeit medicines and tampering, tamper-evident packaging has become an indispensable solution.
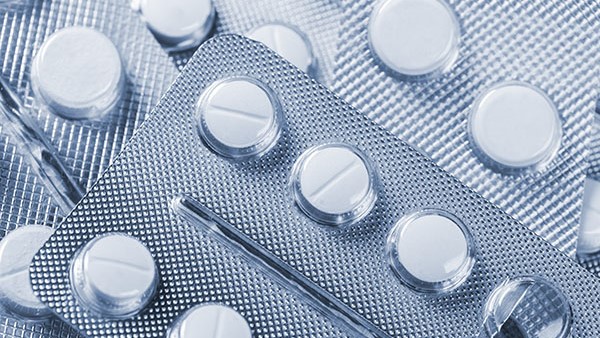
Tamper-evident packaging is a key technology and strategy applied to medicine packaging, aiming to ensure the integrity and authenticity of products during transportation and sale. By employing special security features and technologies such as seal labels, QR codes, and anti-counterfeit markings, tamper-evident packaging effectively prevents tampering, substitution, or replication of the packaging. These security features provide reliable protection, helping pharmaceutical companies and consumers accurately identify genuine medicines and effectively curb the presence of counterfeit medicines.
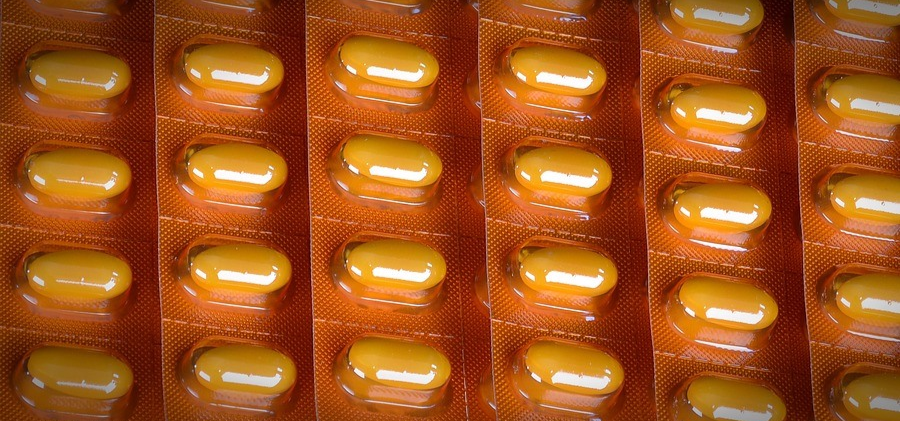
According to the definition by the U.S. FDA, tamper-evident packaging “has one or more indicators or barriers to entry which, if breached or missing, can reasonably provide visible evidence to consumers that tampering has occurred.” Tamper-evident packaging aims to enhance the safety of medicines intended for sale and assist consumers in avoiding potential hazards. Many common medicines, food items, and personal care products have adopted tamper-evident packaging.
Originally, medicine packaging did not incorporate tamper-evident designs. The emergence of tamper-evident packaging can be traced back to the infamous “Tylenol poisoning case.” In 1982, an individual in Chicago tampered with Tylenol capsules by lacing them with cyanide, resulting in at least seven deaths. Since the medicine packaging at the time did not have robust tamper-evident designs, the perpetrator could easily open and reseal the packaging, making it difficult for consumers to detect any anomalies. Following the Tylenol poisoning case, hundreds of imitations occurred in the United States, prompting the inclusion of tamper-evident packaging designs in-laws and industry standards.

The importance of tamper-evident packaging cannot be underestimated. Counterfeit medicines and tampering not only pose a threat to patient’s health but also damage the reputation and profits of pharmaceutical companies. Tamper-evident packaging eliminates the risk to patients from using substandard medicines and prevents counterfeit medicines from compromising the image and profitability of the companies. Furthermore, tamper-evident packaging aids regulatory authorities in their oversight efforts, enhancing the transparency and traceability of the entire medicine supply chain.

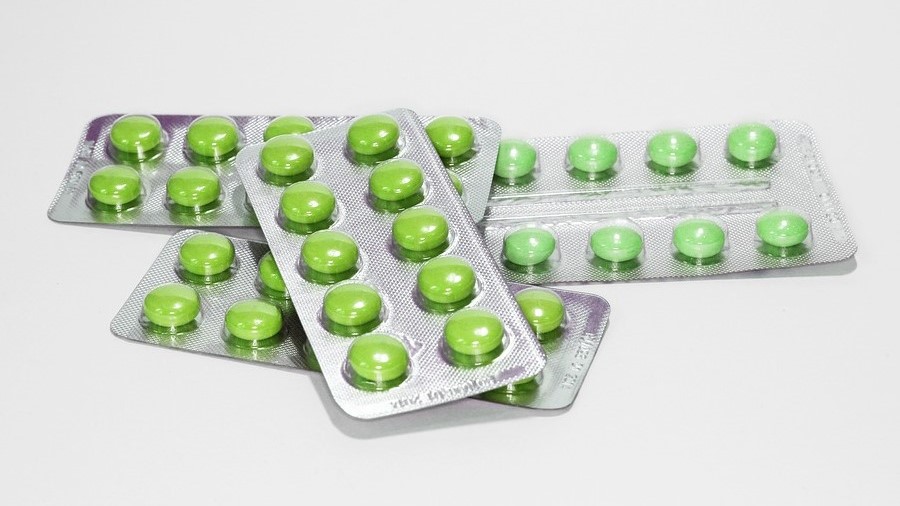
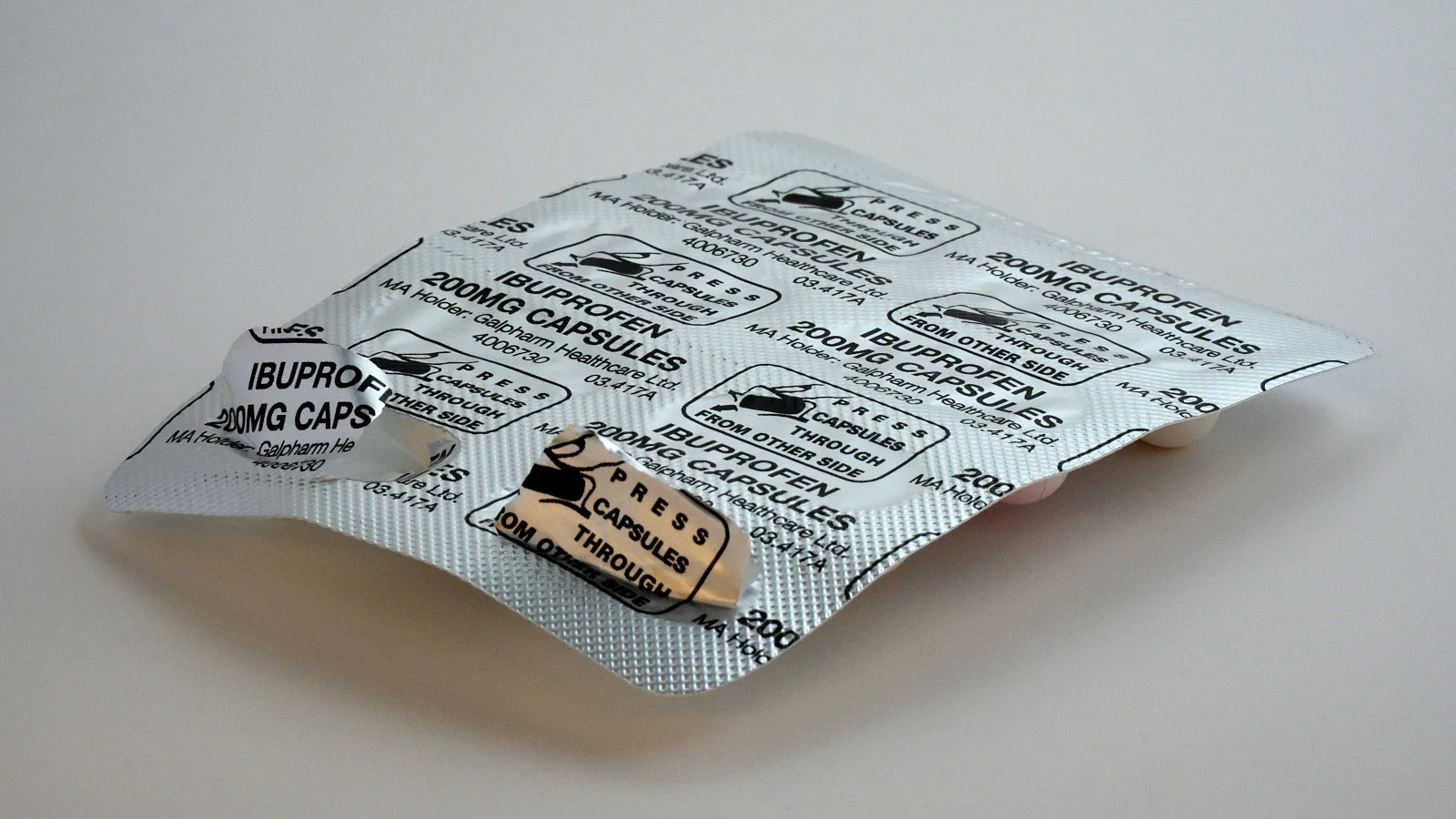
Today, tamper-evident packaging is widely employed in everyday packaging, including food, medicines, and more. Blister packaging is a common form of tamper-evident packaging. In blister packaging, individual doses of medication are sealed within blister cards made of plastic, aluminium foil, or paper materials. Users must break the foil seal to access the medication. This design ensures that consumers can see whether the medicine packaging has been opened, thus helping them avoid potential risks. Additionally, there are other common tamper-evident packaging technologies, such as:
– Seal labels: Applying special seal labels to medicine packaging that leave visible traces when torn or damaged, indicating tampering has occurred.
– QR codes and anti-counterfeit markings: Adding unique QR codes or other anti-counterfeit marks to medicine packaging, allowing consumers to scan the markings using their smartphones or other devices to verify the authenticity and integrity of the product.
– Material selection: Choosing packaging materials with special properties such as anti-counterfeiting, tamper resistance, or difficult replication to enhance packaging security.
– Traceability systems: Establishing traceability systems to track and record medicine packaging throughout the supply chain, ensuring the ability to trace the origins and flow of products, thus reducing the opportunities for counterfeit medicines.
The continuous development and innovation of tamper-evident packaging technologies provide the pharmaceutical industry with powerful tools to combat the threats of counterfeit medicines and tampering. However, as technology advances, perpetrators are also constantly seeking new methods to bypass and overcome tamper-evident packaging. Therefore, pharmaceutical companies and regulatory authorities need to remain vigilant and continuously update and improve tamper-evident packaging technologies to ensure the integrity of medicines and the safety of consumers.

Click on the image to see more
As a professional pharmaceutical packaging machinery manufacturer, Hoping Machinery is dedicated to providing high-quality and efficient packaging solutions to pharmaceutical companies. Hoping Machinery’s blister packaging machine, cartoning machine, and pillow packaging machine, among other products, incorporate advanced technology and innovative designs, enabling the automated high-speed production of medicine packaging while integrating tamper-evident features. Through close collaboration with clients, Hoping Machinery strives to deliver customized solutions based on the unique characteristics and packaging requirements of different medicines, ensuring the safety and effectiveness of medicine packaging.

 VR
VR